Abstract
Background Acquired resistance to immunomodulatory drugs (IMiDs)/cereblon (CRBN) E3 ligase modulators (CELMoDs) is a major challenge in multiple myeloma (MM) treatment. These drugs bind to the CRBN component of the CRL4CRBN E3 ubiquitin ligase and designate a new set of substrates for degradation via the proteasome. Generation of resistance is frequently associated with decreased CRBN expression and this is due to genetic alteration in ~1/3rd of patients. Alternative drivers of the low CRBN state, and other mechanisms of resistance, need to be elucidated. To tackle this complex problem, MM models with acquired IMiD/CELMoD resistance were generated and multiomics analysis performed.
Methods IMiD/CELMoD resistant human MM cell lines were generated by treating MM1s and H929 cells with lenalidomide (Len), pomalidomide (Pom) or iberdomide (Iber) at ~10x GI50 concentration for ~12 weeks until resistance was achieved. Resistant cell lines and controls were characterised by whole exome sequencing (WES), RNA-Seq and proteomics. A genome-wide loss-of-function CRISPR screen (Brunello library) was carried out in Iber-resistant MM1s; gene effect scores were calculated with the Chronos model and compared to parental MM1s using data from the Broad Institute DepMap portal. Pathway analysis was performed using g:Profiler.
The Membrane Bound Transcription Factor Peptidase, Site 1 (MBTPS1) inhibitor PF-429242 was used to inhibit activation of the Sterol Regulatory-Element Binding Protein (SREBP) pathway and the effect on cell viability measured using CellTiter-Blue®. The Multiple Myeloma Research Foundation CoMMpass database was used to explore the correlation between mRNA expression of SREBP pathway genes in newly diagnosed patients and progression-free survival (PFS).
Results All models were resistant to the IMiD/CELMoD with which they were generated (to 20-100x the GI50 concentration) and exhibited cross-resistance to other IMiDs/CELMoDs. Functional assays showed that well-characterised effects of IMiD/CELMoD treatment, e.g. degradation of Ikaros/Aiolos, were abrogated. WES showed Pom-resistant MM1S and Pom- and Iber-resistant H929 cells had acquired mutations in CRBN predicted to have a high impact on function. Len-resistant H929 cells had new copy number loss at the CRBN locus but Len- and Iber-resistant MM1s cells had no genetic changes in CRBN. CRBN protein expression was reduced in all resistant lines compared to control (log2 fold changes (FCs) by proteomics ranging from -0.25 to -1.95, adj p <0.05). Together these models display diverse resistance mechanisms, reflecting the clinical picture.
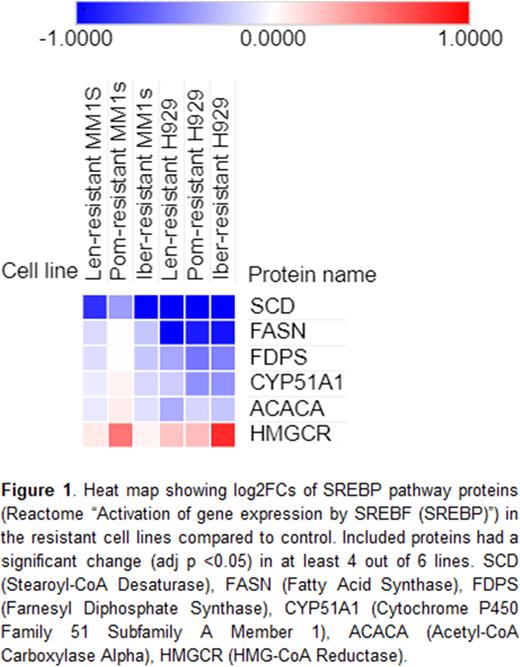
Proteomic analysis of the resistant lines identified key changes in the SREBP pathway. The proteomes of the resistant lines were heterogeneous and the only pathway with common significant enrichment was SREBP/fatty acid metabolism (on analysis performed per cell line of proteins with significantly decreased expression). Stearoyl-CoA Desaturase (SCD), a key effector of the SREBP pathway, was one of only 5 proteins with significantly altered expression in all 6 cell lines compared to control. SCD had log2FCs ranging from -0.37 to -1.50 (adj p <0.05). Other key pathway members are shown in Figure 1.
A genome-wide CRISPR screen using Iber-resistant MM1s cells identified potential new dependencies (gene effect score <-1 in resistant cells and >-0.5 in parental cells) in 47 genes including SCD and MBTPS1. MBTPS1 is critical for activation of the SREBP pathway and demonstrated one of the largest changes in gene effect (-1.4 vs -0.3).
The activity of PF-429242, an inhibitor of MBTPS1, was explored in the resistant lines. A significant difference in GI50 between Iber-resistant H929 cells and control was found (1uM and >10uM respectively) with greater activity in the resistant cells. The same pattern was observed with the other resistant H929 cell lines, but not the resistant MM1s. Other pathway inhibitors are being explored.
The expression of genes encoding SREBP pathway components was explored in the CoMMpass dataset. High SCD or MBTPS1 mRNA expression was associated with significantly worse PFS (logrank p<0.01), providing a further rationale for targeting the pathway.
Conclusions Models representing multiple different IMiD/CELMoD resistance mechanisms have unified alterations in the SREBP pathway, highlighting a role in resistance biology and potential novel and targetable vulnerabilities.
Disclosures
Walker:Genentech: Research Funding; Bristol Myers Squibb: Research Funding. Collins:Monte Rosa Therapeutics: Current equity holder in publicly-traded company; Dunad Therapeutics: Consultancy. Pawlyn:Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel support; Celgene/BMS: Consultancy, Honoraria; Abbvie: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal